Logistical solution for blood tests for humans and animals
WOLF
WOLF is a user-friendly, safe test kit and a significant breakthrough that can be used with various types of CMI tests (Cell-Mediated Immunity) for both humans and animals. These tests identify TBC, Q-fever and Chlamydia, for example. By using WOLF, logistical and time restrictions are gone, and labour costs and laboratory investments are reduced. The WOLF test kit opens up closed areas and greatly increases the capacity for CMI tests.
Medical design facilitates CMI tests in remote locations
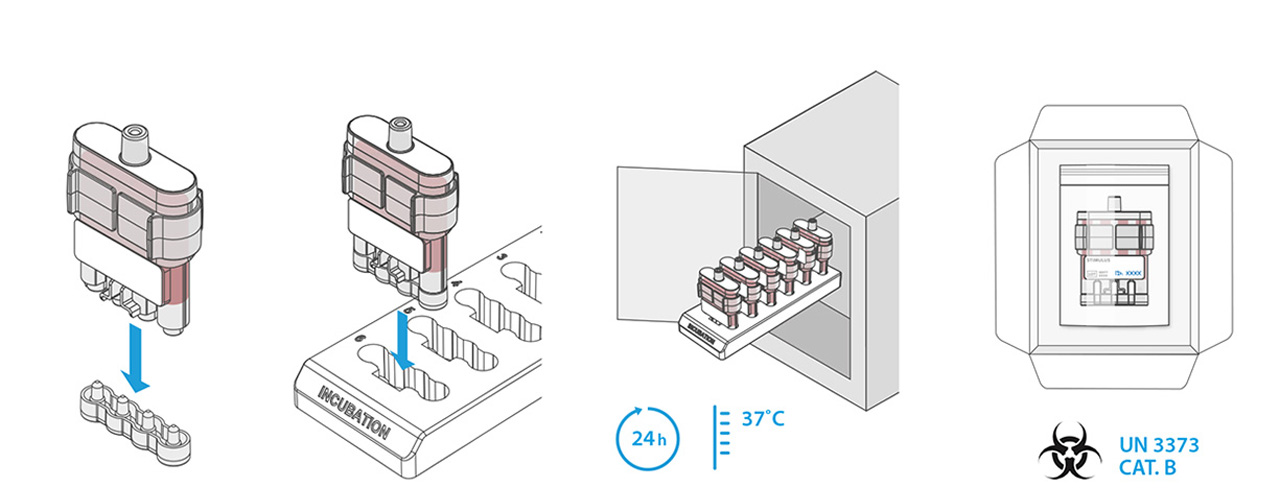
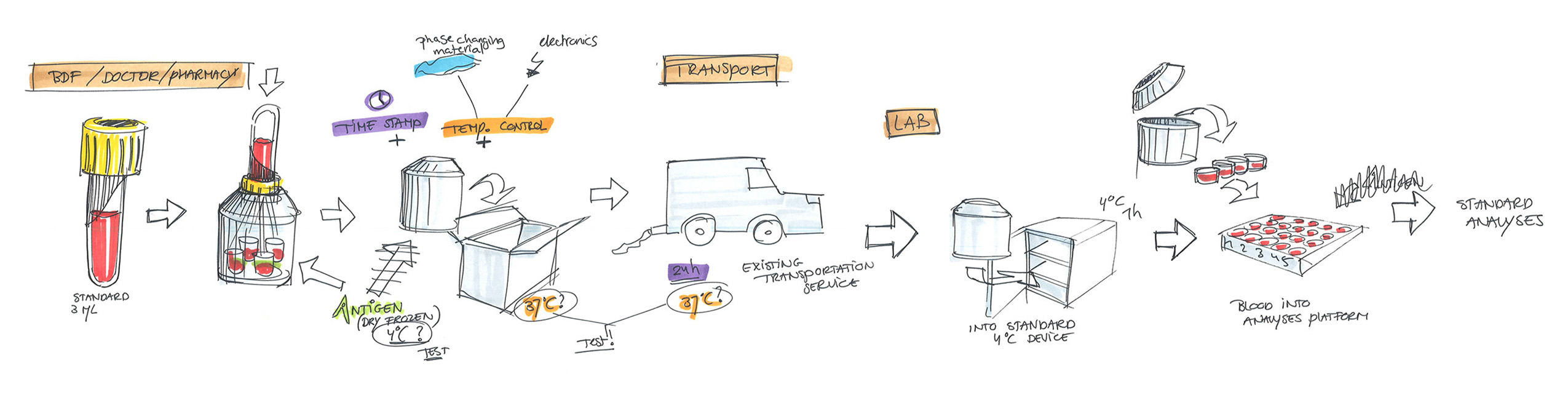
Distance is a limiting factor with the current CMI tests. The test must be done in a laboratory environment within 12 hours after blood sampling. This makes the test expensive and unreliable if this condition cannot be met. A system that carries out the first step of a CMI test close to the location and the time of blood sampling would in theory be the perfect solution for testing in remote/not easily accessible locations.
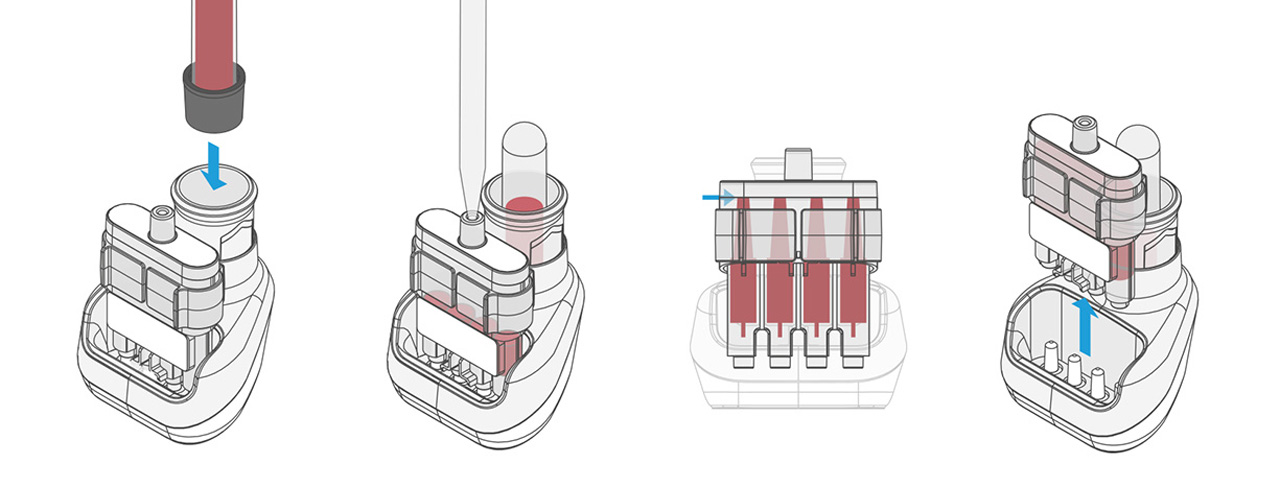
In this first step, an antigen is brought into contact with the blood, producing a reaction. After incubation, the next step is a laboratory analysis, for which time is no longer a limiting factor.
The diagnostic test has been realised within an EU partnership. The catchy name WOLF is a composition of the different cities (Vienna/Wenen, Oss, Leiden and Freiburg) where the project was worked on.
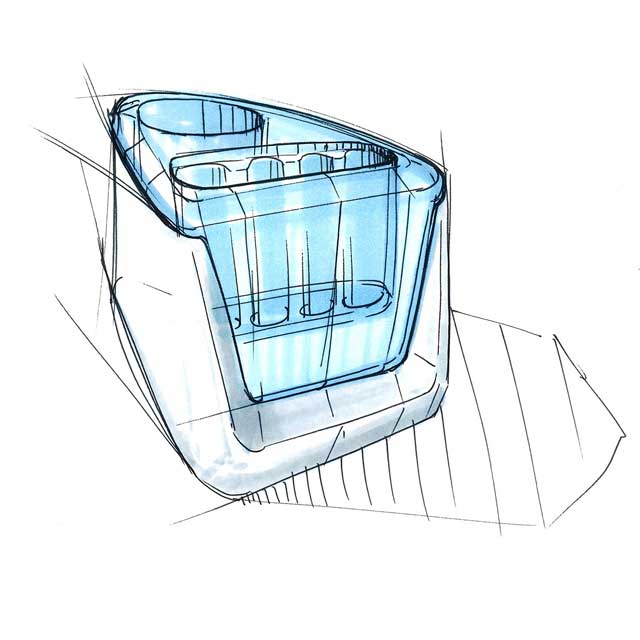


Expert role in the field of medical design
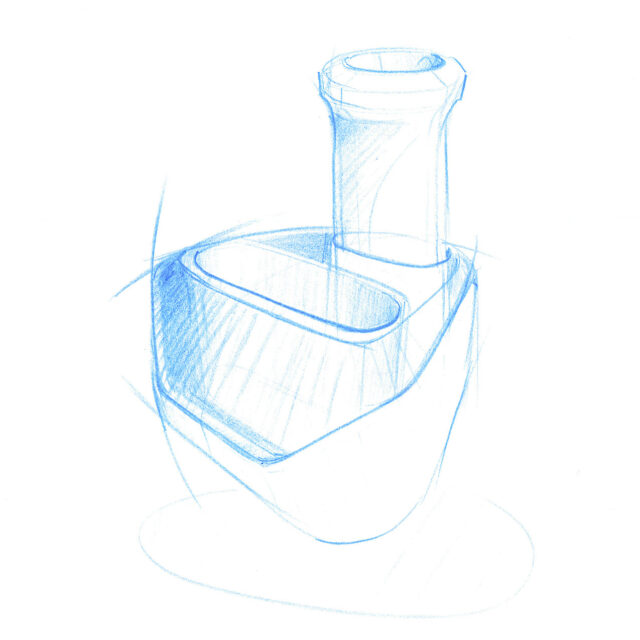
We employed our experience and expertise in the field of medical design in the product design and the development of a procedure and device with which the antigen test can be conducted. User-friendliness and safety form crucial factors.
Our design is an innovative system that divides the blood immediately after sampling equally into different reservoirs containing the reagent and then seals them safely and ready for transport. A patent for the working principle has been applied for.
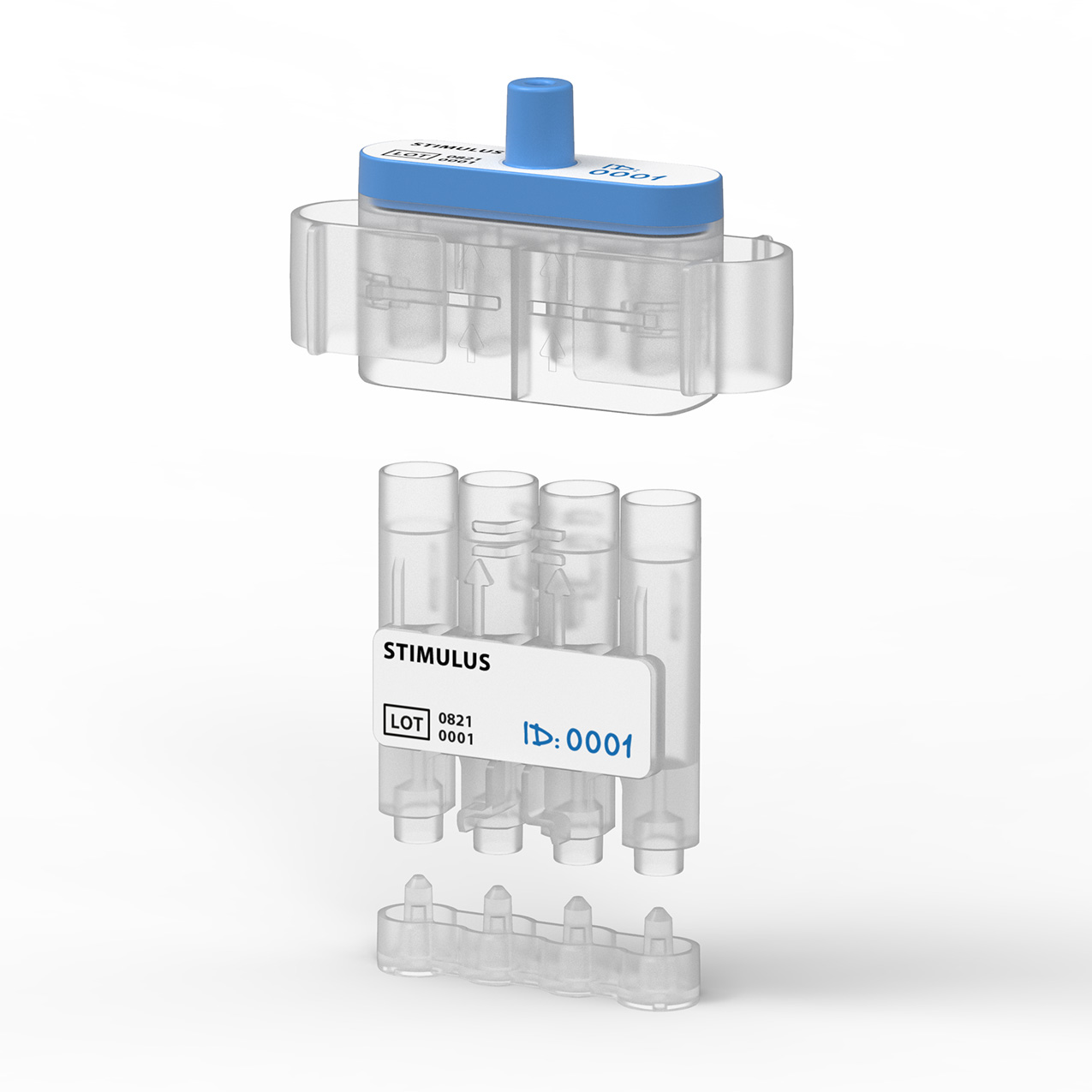
Legislation, FMEA protocols and Fluid dynamics
Medical design demands experience with the development of medical applications and the suitable materials for them. The product solution and specifications must enable a suitable manufacturer to produce cleanly so the product does not affect the test content. During the entire product development process, we comply with the legislation on medical devices for in vitro diagnostics (IVDR). Fluid dynamics play a large role in this project, because blood is an extremely difficult fluid to work with, in terms of both material and safety. This demands a precise and exacting approach and testing based on risk management procedures.